Autologous Hematopoietic Stem Cell Transplantation In Multiple Sclerosis A Phase Ii Trial
Background autologous haematopoietic stem cell transplantation ahsct has been explored as a therapeutic intervention in multiple sclerosis ms over the last two decades.
Autologous hematopoietic stem cell transplantation in multiple sclerosis a phase ii trial. A phase ii trial. Autologous hematopoietic stem cell transplantation in multiple sclerosis. The main objective of our work is to describe the long term results of myeloablative autologous hematopoietic stem cell transplant ahsct in multiple sclerosis patients.
We conducted a multicenter phase ii randomized trial including patients with secondary progressive or relapsing remitting ms. Neurology 2015 2 13 autologous hematopoietic stem cell transplantation in multiple sclerosis. Autologous hematopoietic stem cell transplantation in multiple sclerosis.
A phase ii trial to assess in multiple sclerosis ms the effect of intense immunosuppression followed by. However prospective clinical trials of the most common myeloablative conditioning regimen beam are limited. Autologous hematopoietic stem cell transplantation also known as stem cell therapy or by the acronym ahsct is an emerging yet controversial treatment method for multiple sclerosis ms.
Giovanni l mancardi maria p sormani francesca gualandi albert saiz eric carreras elisa merelli amedea donelli alessandra lugaresi paolo di bartolomeo maria r rottoli alessandro rambaldi maria p amato luca massacesi massimo di gioia luisa vuolo daniela currò luca. A phase ii trial. Haematopoietic stem cell transplantation hsct has been in use for treatment of malignancies since the 1950s 1 2 in the last two decades it has been used for treatment of autoimmune diseases of the nervous system such as multiple sclerosis ms neuromyelitis optica nmo chronic idiopathic demyelinating polyneuropathy cidp and myasthenia gravis mg.
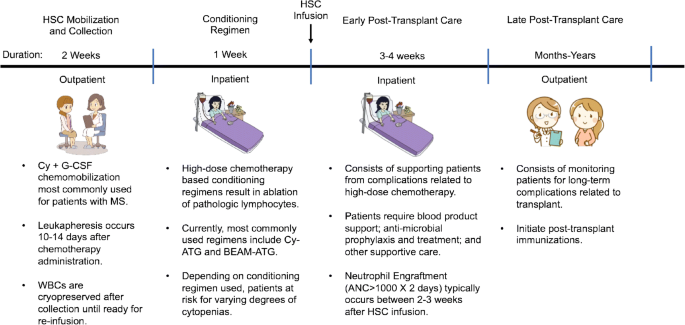
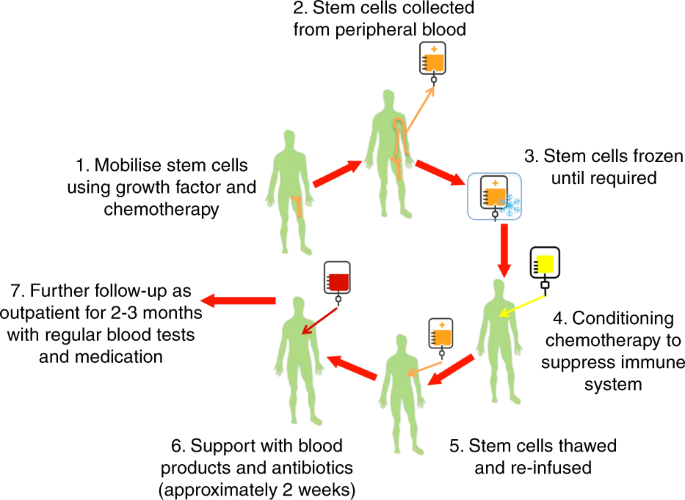
Request pdf autologous hematopoietic stem cell transplantation in multiple sclerosis. Furthermore patient selection optimal chemotherapeutic regimen and immunological changes associated with. Patients that failed to conventional therapies for multiple sclerosis ms underwent an approved protocol for ahsct which consisted of peripheral blood stem cell mobilization with cyclophosphamide and granulocyte colony.
J neurol neurosurg psychiatry. Epub 2018 dec 11. We conducted a multicenter phase ii randomized trial including patients with secondary progressive or relapsing remitting ms with a documented increase in the last.
A phase ii trial.